Diabetic eye care
Diabetic retinopathy (DR) is a chronic progressive, potentially sight-threatening disease of the retinal microvasculature, associated with the prolonged hyperglycaemia of diabetes mellitus and with other diabetes mellitus-linked conditions, such as hypertension.
Diabetes mellitus can cause a variety of eye problems, the most common being DR, which is the most common cause of severe sight impairment among people of working age. Other conditions associated with diabetes and the eye include:
Rubeosis iridis and glaucoma.
Ocular motor nerve palsies.
DIABETIC RETINOPATHY
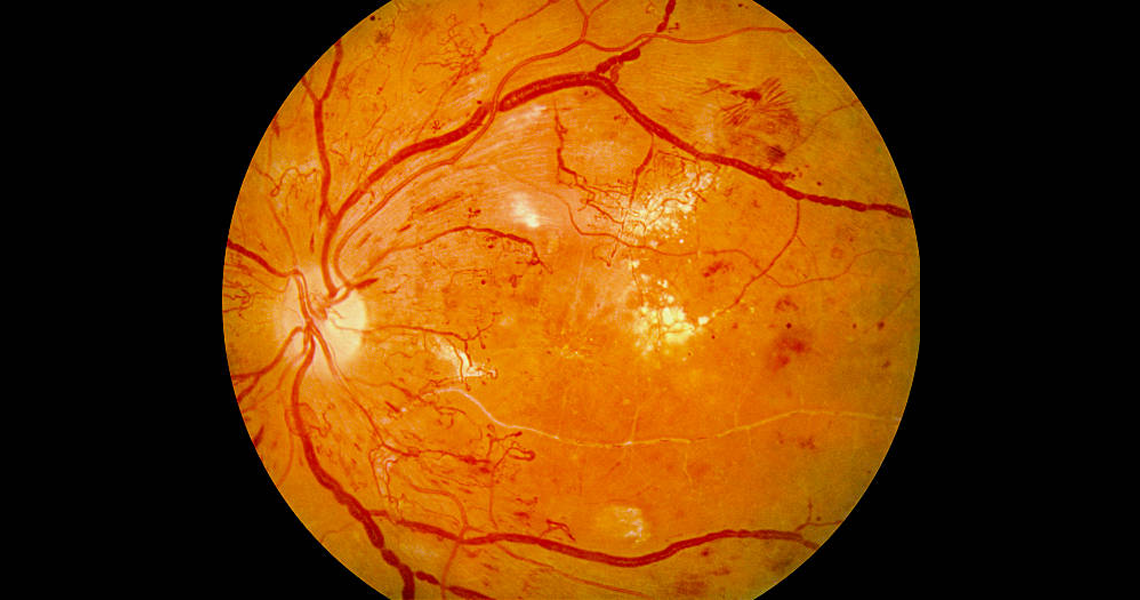
The exact mechanism by which diabetes leads to DR is not fully understood. Microvascular occlusion causes retinal ischaemia leading to arteriovenous shunts and neovascularisation. Leakage results in intraretinal haemorrhages and localised or diffuse oedema. These processes result in the characteristic features seen at various stages of DR:
Microaneurysms - physical weakening of the capillary walls which predisposes them to leakages.
Hard exudates - precipitates of lipoproteins/other proteins leaking from retinal blood vessels.
Haemorrhages - rupture of weakened capillaries, appearing as small dots/larger blots or 'flame' haemorrhages that track along nerve-fibre bundles in superficial retinal layers (the haemorrhage arises from larger and more superficial arterioles).
Cotton wool spots - build-up of axonal debris due to poor axonal metabolism at the margins of ischaemic infarcts.
Neovascularisation - an attempt (by residual healthy retina) to revascularise hypoxic retinal tissue.
The classification of DR is based on which part of the retina is affected and the degree of pathology seen on slit-lamp examination of the eye. It is not necessarily correlated to the degree of vision, which may be almost normal until the very late stages of the disease when little can be done to save it. Broadly speaking, DR falls into two types:
Diabetic retinopathy:
Background (mild) non-proliferative DR: at least one microaneurysm.
Moderate non-proliferative DR: microaneurysms or intraretinal haemorrhages ± cotton wool spots, venous beading, intraretinal microvascular abnormalities (IRMAs).
Severe to very severe non-proliferative DR(sometimes referred to as pre-proliferative disease), as above: a minimum number of these features are required in a minimum number of retinal quadrants to define severe or very severe disease.
Non-high-risk proliferative DR: new vessels on the disc (NVD) - or within one disc diameter of it or new vessels elsewhere (NVE).
High-risk proliferative DR: large NVD or NVE (defined by comparing to the optic disc surface area) or presence of pre-retinal haemorrhage. In advanced disease, there may also be an accompanying retinal detachment.
Diabetic maculopathy:
Focal or diffuse macular oedema: areas of leakage which may be well circumscribed or diffuse.
Ischaemic maculopathy: the clinical appearance may be relatively normal but the visual acuity has dropped and ischaemia is seen on fluorescein angiography.
Clinically significant macular oedema (CSMO): there may be thickening of the retina and hard exudates which, when found within a specific distance of the fovea or when found to be above a certain size, define CSMO.
EPIDEMIOLOGY
In type 1 diabetes, microaneurysms start to appear after five years in 25% of cases, affect half of cases at 10 years and nearly all patients after 20 years. Proliferative retinopathy, as defined by a formation of new vessels, appears after 10 years and affects about 40% after 20 years. Maculopathy follows a similar pattern, finally affecting 10-20% of cases.
In type 2 diabetes, these changes may be found at diagnosis because subclinical hyperglycaemia may have been present for a prolonged preceding period. Over 25 years, there is a significant cumulative rate of progression to DR (83%), to diabetic macular oedema (29%) and to CSMO (17%).
RISK FACTORS
Progression of retinopathy is associated with the severity and length of time that hyperglycaemia If diabetes is diagnosed before the age of 30, the incidence of DR after 10 years is 50%, rising to 90% after 30 years. There is no set glycaemic threshold that will predict the presence or otherwise of DR.
Hypertension and other cardiovascular risk factors can influence the onset and progression of retinopathy.There is marked individual variation in susceptibility to retinopathy for a given vascular risk profile.
Renal disease, as evidenced by proteinuria and elevated urea/creatinine levels, is an excellent predictor of the presence of retinopathy.
Pregnancy can be associated with a rapid progression of DR, particularly if:
There is severe baseline retinopathy.
There is poor glycaemic control at conception, during pregnancy or in the postpartum period.
There is rapid improvement of diabetic control.
The diabetes has been present for a long time.
The patient is hypertensive (chronic or pregnancy-induced).
It is thought that intraocular surgery may possibly increase the risk of progression of DR.
SCREENING AND REFERRAL
Adults with type 1 or type 2 diabetes[7, 8]
Arrange or perform eye screening at or around the time of diagnosis for adults with type 1 or type 2 diabetes. Arrange repeat of structured eye screening annually. Use mydriasis with tropicamide when photographing the retina, after prior informed agreement following discussion of the advantages and disadvantages. Discussions should include precautions for driving.
Use a quality-assured digital retinal photography programme using appropriately trained staff. Perform visual acuity testing as a routine part of eye screening programmes. Depending on the findings, follow structured eye screening by routine review in one year, earlier review or referral to an ophthalmologist.
Arrange emergency review by an ophthalmologist for:
Sudden loss of vision.
Rubeosis iridis.
Pre-retinal or vitreous haemorrhage.
Retinal detachment.
Arrange rapid review by an ophthalmologist for new vessel formation.
Refer to an ophthalmologist in accordance with the National Screening Committee criteria and timelines (referred to the hospital eye services within four weeks of the result) if any of these features are present:
Referable maculopathy:
Exudate or retinal thickening within 1 disc diameter of the centre of the fovea.
Circinate or group of exudates within the macula (the macula is defined here as a Circle centred on the fovea, with a diameter the distance between the temporal border of the optic disc and the fovea).
Any microaneurysm or haemorrhage within 1 disc diameter of the centre of the fovea, only if associated with deterioration of best visual acuity to 6/12 or worse.
Referable pre-proliferative retinopathy (if cotton wool spots are present, look carefully for the following features; however, cotton wool spots themselves do not define pre-proliferative retinopathy):
Any venous beading.
Any venous reduplication.
Any intraretinal microvascular abnormalities.
Multiple deep, round or blot haemorrhages.
Any large, sudden unexplained drop in visual acuity.
Children and young adults
Monitoring for DR should begin at 12 years of age for both type 1 and type 2 diabetes.
Consider referring children and young people with type 2 diabetes who are younger than 12 years to an ophthalmologist for retinal examination if blood glucose control is suboptimal.
INVESTIGATIONS
Fundus photography and examination are sufficient for most patients.
However, optical coherence tomography is playing an increasingly important role in assessing the presence of macular oedema (and then recording its progression over several visits) and fluorescein angiography may be helpful where CSMO is present (to guide laser treatment) and where the vision is unexpectedly poor (to assess for macular ischaemia).
MANAGEMENT
Primary prevention
Glycaemic control:
Optimal glycaemic control (usually aiming to bring HbA1c levels to <7%, ideally around 6.5%) is associated with improved long-term outcomes and delayed progression of retinopathy.
However, in some cases, particularly with pre-proliferative and proliferative retinopathy, intensive glycaemic control (eg, HbA1c at 6.0%) can initially bring on a decompensation and worsening of symptoms and signs and is also associated with increased mortality.
Blood pressure control:
Good control of blood pressure (target: 140/80 mm Hg or lower) reduces the progression of DR significantly and is associated with a 32% reduction in diabetes-related deaths.[1]
If possible, aim for systolic ≤130 mm Hg in those with established retinopathy and/or nephropathy.
Specific therapies blocking the renin-angiotensin system (RAS) may have additional benefits, particularly for mild retinopathy, but should be discontinued during pregnancy.
Lipid control:
Lipid-lowering therapy has been shown to reduce the risk of progression of diabetic retinopathy, particularly macular oedema and exudation.
Consider adding fenofibrate to a statin for non-proliferative retinopathy in type 2 diabetes.
A healthy, balanced diet and exercise should be discussed with the patient.
Smoking cessation.
There have been more recent trials looking at what can be done for those individuals in whom glycaemic and blood pressure control is good but DR is progressive:[11]
The Diabetic Retinopathy Candesartan Trials (DIRECT) looked at the effect of candesartan, an angiotensin-II receptor antagonist, on these patients and found somewhat equivocal results. In patients with type 1 diabetes, there was a modestly reduced incidence of retinopathy by 18% but there was no effect on the progression of existing retinopathy. In patients with type 2 diabetes, there was a significantly increased regression of existing retinopathy by 34% and progression was reduced by 13% (this last finding was not statistically significant).
The Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study was more promising, showing that fenofibrate, a lipid-lowering fibrate, reduced the need for laser treatment of sight-threatening DR (either for macular oedema or proliferative retinopathy) by 31% over five years.
Laser treatment can arrest the progression of DR but is unlikely to restore any lost vision.
Treatment can be targeted on to specific areas (focal treatment) or delivered over the entire periphery of the retina (panretinal photocoagulation (PRP)) where 1,200-1,600 burns may be placed on the retina over 2-3 sessions. The choice depends on the nature of the DR: macular oedema is treated with focal laser burns whereas retinopathy is more amenable to PRP. If there is both retinopathy and maculopathy, macular oedema is often treated first and separately before treatment with PRP.
Laser treatment is carried out in a laser treatment clinic on an outpatient basis. At a later date, the areas of laser treatment are easily identifiable as well-demarcated pale spots with distinct dark-brown centres - this can be helpful if the patient cannot remember if they have previously had treatment!
The decision as to whether to carry out laser treatment is not always clear-cut (eg, asymptomatic patients with CSMO but no visual loss).
Intravitreal steroids[12]
A large trial has demonstrated that intravitreal steroids were initially more effective than treatment with laser photocoagulation but that two years post-treatment, eyes treated with laser actually had better visual acuity and less maculopathy.
Intravitreal triamcinolone appears to reduce CSMO and improve visual acuity in more advanced cases. It may be used as primary or adjunctive therapy. The effect is maximal after a week but may last up to six months.
The mechanism of action of corticosteroids is not fully understood.
This treatment modality is associated with complications (see 'Complications', below).
Fluocinolone acetonide:
Fluocinolone acetonide intravitreal implant is a corticosteroid that has anti-inflammatory and anti-vascular endothelial growth factor properties.
Fluocinolone acetonide intravitreal implant is recommended by the National Institute for Health and Care Excellence (NICE) as an option for treating chronic diabetic macular oedema that is insufficiently responsive to available therapies but only if the implant is to be used in an eye with an intraocular (pseudophakic) lens.[13]
Anti-vascular endothelial growth factor treatments
In recent trials, anti-vascular endothelial growth factor drugs have shown a definite but small benefit compared to other current therapeutic options for the treatment of diabetic macular oedema.[14]
Pegaptanib, bevacizumab and ranibizumab have all been investigated with promising results.[2]
Currently, both price and the frequency of attendance required (both for the injections and for the follow-up) limit the use of these treatments in clinical practice. Ranibizumab is currently not recommended by NICE for the treatment of visual impairment due to diabetic macular oedema.[15]
Surgery
A vitrectomy (removal of the vitreous) may be required following an intravitreal bleed in proliferative DR.
Not only does it physically remove the blood to allow vision through a clear medium but any retinal detachment can also be repaired. Intra-operative PRP reduces the stimulus for neovascularisation.
COMPLICATIONS
The main complication of DR is visual loss secondary to:
Macular ischaemia.
PROGNOSIS
Background retinopathy will eventually progress to the more severe forms in the majority of individuals. If left untreated, 50% of those with proliferative DR will lose their sight within two years and 90% risk losing any useful vision after 10 years.
Patients who undergo treatment have their risk of moderate visual loss reduced from 30% to 15% over the subsequent three years. Those who have PRP have their risk of severe visual loss reduced by 50%, compared with untreated individuals with a similar severity of disease.
OTHER EYE CONDITIONS ASSOCIATED WITH DIABETES
Cataracts
A classic diabetic cataract is rare. The cataract is manifest as snowflake opacities occurring in the young person with diabetes. It may resolve spontaneously, or mature.
More commonly, an age-related cataract is precipitated in the diabetic patient, to form earlier than it would have done otherwise.
Eye conditions less commonly associated with diabetes
Premature presbyopia and other refractive errors due to the reduced pliability of the lens secondary to an altered metabolism.
Rubeosis iridis describes the process when severe ischaemia causes neovascularisation to such an extent that the vessels grow forward and over the iris. The vessels may be seen as large individual entities or else give the iris a generally red appearance. If they block the peripheral trabecular meshwork (through which most of the aqueous drains) on the way, they may precipitate acute glaucoma, which needs urgent treatment
Occasionally, ocular motor nerve palsies occur, presumably due to the damage of the microvascular supply of these cranial nerves. Patients should be presumed to have an intracranial mass until proven otherwise via imaging. If it is truly a palsy related to diabetic microvasculopathy, it often resolves over a period of months but orthoptic input may be needed.
Other eye conditions more commonly found in people with diabetes include dry eyes, corneal abrasions, anterior uveitis, ocular ischaemic syndrome, papillitis and orbital infections.
Corneal abnormalities may also be found in these patient groups.
Asteroid hyalosis is a condition characterised by little white flecks seen in the vitreous. It can occur for a number of reasons and is usually asymptomatic. Unless very severe and affecting vision, it is left alone.
